Elevating clinical trial quality and compliance with BSI eTMF
by Pauliina Ranne – Senior Consultant
 Overview
Overview
As the clinical trial landscape continues to evolve, the adoption of a electronic Trial Master File (eTMF) is becoming the norm rather than the exception. Especially as the health authorities are swiftly moving towards remote and hybrid inspections, which consequently puts sponsors in a situation where they might face inspections more often. Therefore, they are forced to place stronger emphasis on keeping their trial documentation consistently inspection ready. BSI eTMF offers a solution that can support sponsors in these endeavors by significantly enhancing document management practices and ensuring trial document compliance with regulatory standards.
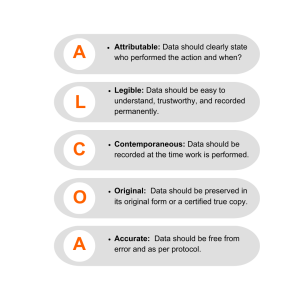
By moving from traditional paper-based methods to an eTMF system, organizations can streamline document management processes, facilitate real-time access for various parties, and ensure better regulatory adherence by adhering to the ALCOA criteria—Attributable, Legible, Contemporaneous, Original, and Accurate.
eTMF systems enable the centralization of all trial-related documents, making it easier to organize, search, and retrieve information efficiently. This consolidation reduces the time and effort spent on manual filing and document retrieval, significantly improving workflow efficiency.
Additionally, eTMF systems support real-time updates, allowing authorized personnel to access the most current data from any location, which enhances collaboration and decision-making processes. Furthermore, these systems are designed with compliance in mind, incorporating features such as audit trails, electronic signatures, and automated compliance checks to ensure that all documentation meets strict regulatory standards.
 How does BSI eTMF support document management quality and compliance?
How does BSI eTMF support document management quality and compliance?
BSI Life Sciences eTMF offers a highly intuitive and configurable platform for optimizing the management of clinical trial documents for any type of life science sponsor and CROs.
With the following features, the solution covers the most important aspects of clinical trial document management which support companies to remain ready for audits and inspections.
 Document Management
Document Management
The system supports setting up the document plan following the preconfigured CDISC TMF Reference Model or customizing it to individual needs, also per trial or by sponsor preferences if needed.
With BSI eTMF you can give sites restricted access via a site portal to keep the TMF and the Investigator Site File (ISF) in one place, exchanging essential documents like VC, 1572s etc. You can define the essential metadata, study, and site number etc., customize with additional fields, and organize documents in folders.
To streamline the document management process, it is easy to create document plans (with automatic placeholders and configured templates as needed), set up document review and approval workflows with enhanced alerting capability, and configure reports to track completeness of documents.
 Monitoring and Reporting
Monitoring and Reporting
BSI eTMF empowers users with flexible reporting, allowing personalized document reports through easy configuration and export to Word or Excel. It provides efficient document searches with Global Search, completeness status insights (e.g. on site level), and general document reports for diverse user groups.
Users can export documents in ZIP files according to the Trial Master File structure, maintaining security with limited access for inspectors and auditors.
 Regulatory Compliance
Regulatory Compliance
Ensuring regulatory compliance is paramount in clinical trials, and BSI eTMF addresses this by adhering to industry standards and regulatory requirements such as from 21 CFR Part 11, ICH-GCP E6(R2) and GDPR (General Data Protection Regulation).
The system enables users to electronically sign documents and tracks all activities in the audit trail, providing a transparent and comprehensive history of document changes, approvals, and access. This feature facilitates compliance during regulatory inspections and enhances the integrity of trial documentation.
 User Access
User Access
Granting and managing access to BSI eTMF for study partners, inspectors, and auditors is straightforward. Through the BSI Portal, sites can obtain limited yet convenient access to the system, enabling them to efficiently upload crucial documents directly to the eTMF. This streamlined process significantly enhances task completion time and ensures data security.
Whether you’re a small sized life science business or a larger company, BSI eTMF will not only help you ensure regulatory compliance but also improve operational efficiency and collaboration across teams. Supported by BSI’s structured implementation process, you’ll be able to move your clinical trial documentation into the new system seamlessly and continue the pursuit of achieving excellence in clinical documentation and trial management.
